Better Oocytes in our Sights
7 min read
Oocyte quality affects the conception rates in dairy cows. An oocyte is the egg that, when fertilised by sperm, produces offspring. It matures within follicles on the ovary and its quality is essential for early embryo survival. A major challenge is that many dairy cows lose their embryos in the first week after insemination, often due to poor oocyte quality. The quality of an oocyte can be influenced by the cow's health, nutrition, and environment. DairyNZ researchers are exploring ways to enhance oocyte quality, aiming to provide practical solutions to New Zealand dairy farmers to boost conception rates.
DairyNZ researchers have been investigating ways to improve oocyte quality, with the end goal of lifting conception rates in New Zealand dairy herds. Find out what we’ve discovered and what’s next.
Dairy cows experience a high rate of embryonic loss in the first week of pregnancy. Oocyte (egg) quality is an important determinant of embryo survival through this period.
The developing oocyte is sensitive to changes within the follicle that nurtures it.
Lactating cows with a high Fertility Breeding Value (BV) produce better oocytes and have a different follicular environment than cows with low Fertility BV.
Improved oocyte quality and conception rate are features of cows with higher genetic fertility.
DairyNZ’s aim is to find practical solutions for New Zealand dairy farmers to increase oocyte quality, and therefore conception rates, in their dairy cows.
DairyNZ post-doc scientist Charlotte Reed (left) and senior research technician Olivia Jordan at work during the first oocyte trial.
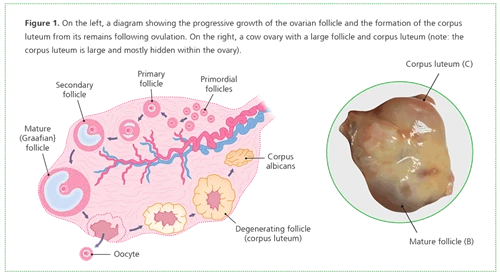
An oocyte is the egg which is fertilised by a sperm cell to produce offspring. Oocytes mature within follicles on the ovary. As shown in Figure 1, these grow from small follicles (A) to large, mature follicles with a central fluid-filled cavity (B).
During each reproductive cycle, cows will typically ovulate one mature follicle following oestrus. The ovarian follicle ruptures, releasing the oocyte, which passes into the oviduct to meet with sperm. Following fertilisation, nutrient stores within the oocyte are essential to support the early pregnancy, until the embryo can assume control over its own development. The remains of the ruptured follicle form the corpus luteum (C) which secretes progesterone, the hormone required to maintain the pregnancy.
Dairy cows experience a high rate of embryonic loss in the first week of pregnancy. While 80 percent to 90 percent of cows have a fertilised oocyte following insemination, almost a third of these fertilised oocytes are not viable seven days later1. This very early stage of pregnancy relies on nutrient stores within the oocyte to support early development. Therefore, oocyte quality is an important determinant of embryo survival through this period2.
The high incidence of embryonic loss in New Zealand dairy cows during the first week after insemination is likely to be due, at least in part, to oocytes of poor quality. These oocytes are unable to sustain the embryo through this critical phase.
While it may appear that the ovarian follicle isolates and protects the oocyte from the external world, the oocyte is in fact very sensitive to environmental changes3. Alterations in blood metabolites and hormones, due to changes in the environment, health and nutrition, are generally reflected in the follicular fluid within developing follicles4. These changes in follicular environment affect the quality of the oocyte within.
Dairy cows are routinely exposed to factors – notably negative energy balance in early lactation, when body tissue is mobilised to meet energy demand, and metabolic or inflammatory diseases, such as mastitis or uterine infection – that reduce oocyte quality.
Furthermore, these conditions may affect not only the oocytes within the larger follicles, but also those in smaller primary and secondary follicles5 (Figure 1).
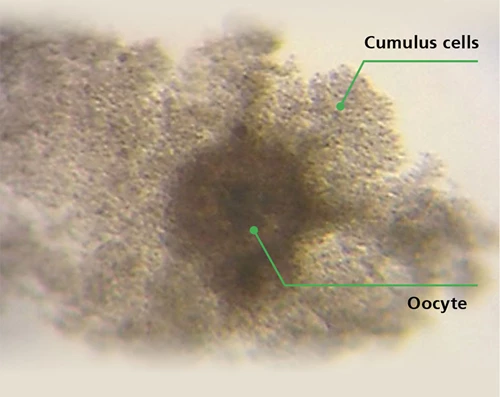
A cow oocyte and cumulus cells. Cumulus cells provide the oocyte with nutrients and coordinate its maturation.
Bovine follicles take 80 to 100 days to develop to the ovulatory stage, once activated to grow5. This means the reproductive consequence of negative energy balance or disease may still be seen months later, when oocytes exposed to detrimental conditions early in their development reach ovulation6. Therefore, during mating, most cows will be ovulating oocytes that were exposed to relatively poor metabolic conditions in early lactation. This may affect their fertility.
At DairyNZ, we’ve been investing farmers’ levy in research* to explore ways in which oocyte quality, and therefore conception rates, could be improved. Our first task was to characterise follicular environments that can support the development of a high-quality oocyte. We compared oocyte quality and the corresponding follicular environment between dairy cattle with very high (+5) or very low (-5) Fertility BV, as non-lactating heifers, and again during the first lactation. We recovered the oocyte, their supporting cumulus cells and a sample of the fluid within the ovarian follicle from cows and heifers in heat, just before ovulation.
To assess oocyte quality, we measured the expression of genes, in the oocyte and its supporting cumulus cells, that are associated with good or poor oocyte quality. High-fertility lactating cows had greater expression of genes (VCAN and PDE8A) associated with higher oocyte competency and that have been linked to a greater proportion of live births in humans7, 8.
This indicates that oocytes from high-fertility cows are of better quality and have a greater chance of establishing a successful pregnancy than those from low-fertility cows.
A partially dissected ovarian follicle.
Heifers generally have better fertility and higher-quality oocytes than lactating cows9. There was no difference in oocyte quality between high-fertility and low-fertility non-lactating heifers. So, oocyte quality may not limit the fertility of yearling heifers with low genetic fertility.
Our research found differences between high- and low-fertility cows, but not non-lactating heifers, in the composition of fluid taken from within the follicle. Compared with low-fertility cows, high-fertility cows had lower non-esterified fatty acids (NEFA) and amino acid concentrations, and altered hormone concentrations in their follicular fluid. These differences were not apparent between the high- and low-fertility heifers.
NEFA are produced when fat stores are broken down for energy. While they provide a valuable source of energy, NEFA impair cell function at high concentrations10. NEFA concentration in the bloodstream is reflected in the follicular fluid, where it is particularly harmful to the follicle and oocyte within, affecting hormone production, metabolism and maturation. High-fertility cows had lower NEFA concentrations within their follicles, creating a better environment for the oocyte. This may partially explain the high quality of their oocytes, compared to those from the low-fertility cows.
The elevated follicular NEFA concentrations in the low-fertility group don’t appear to reflect a greater breakdown of bodyfat, as there was no difference in blood concentrations of NEFA between the low- and high-fertility cows. Cows are thought to have some ability to protect their follicles and oocytes against rises in circulating NEFA, as follicular NEFA concentrations are typically lower than those in blood11. The high-fertility group had a lower ratio of follicular NEFA concentrations relative to their plasma concentrations. This tells us that the mechanisms protecting the oocyte from high-circulating NEFA may be more effective in these cows.
Amino acids in the follicular fluid are used by the oocyte and follicular cells for a wide range of processes, including energy production, cell signalling and protein synthesis12. Follicular concentrations of several amino acids were higher in low-fertility cows, which may indicate they have a reduced ability to use these amino acids.This may be associated with the high NEFA concentrations in their follicles, as this can interfere with cellular metabolism.
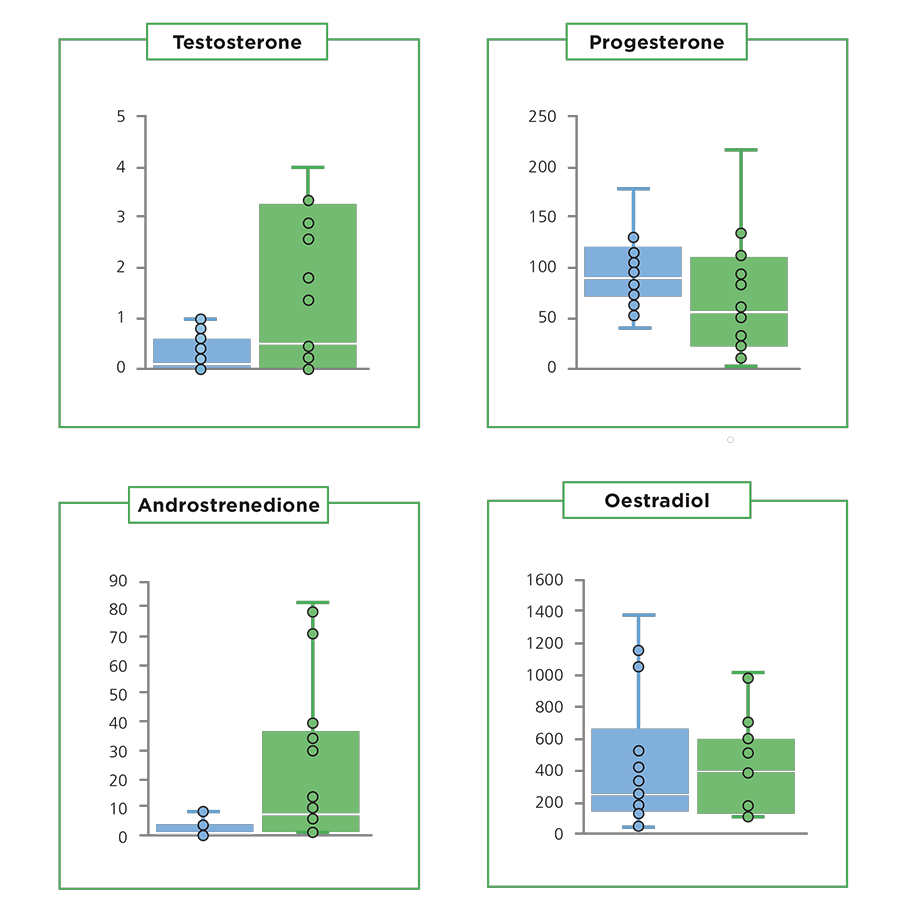
Differences in follicular hormone concentrations between the high- and low-fertility cows may indicate that low-fertility cows have a delayed response to the signal to ovulate. The ovulation process is associated with coordinated changes in hormone production, whereby the concentrations of oestradiol and androgens decrease and progesterone increases. In our study, although follicles were sampled shortly before ovulation, the low-fertility cows still had high androgen (testosterone and androstenedione) and lower progesterone concentrations (Figure 2). As increased follicular progesterone indicates healthy advancement of the ovulatory process, the low-fertility cows may take longer to ovulate relative to the onset of oestrus. Delayed ovulation relative to oestrus and insemination is less likely to result in a pregnancy.
Figure 2. Hormone concentrations (nanograms per millilitre ± interquartile range) in the follicular fluid of cows with high (blue boxes) or low (green boxes) Fertility Breeding Value.
The differences we’ve identified in the follicular environment are now being used to design artificial environments to mature oocytes in the laboratory. We will then assess how oocyte function is affected by the differences we observed in amino acids, hormone or NEFA concentrations. Our aim is to understand how changes in the follicular environment have an impact on oocyte function. Ultimately, we want to find practical solutions for New Zealand dairy farmers to increase oocyte quality, and therefore conception rates, in their dairy cows.
This project was funded by a partnership (DRCX1302) between the Ministry of Business, Innovation and Employment and dairy farmers, through DairyNZ Inc., and includes AgResearch core funding. The trials ran from 2016 to 2018.
References
Scientist
Now’s the perfect time to check in, plan, and set up for a strong season. We’ve pulled together smart tips and tools to help you stay ahead all winter long.
Whether you prefer to read, listen, or download handy guides, we’ve got you covered with trusted tools to support your journey every step of the way.